The Psychedelic Revolution Will Require Human-Relevant Models
Written by Donya Mand, M.D.
October 2024

In August, the U.S. Food and Drug Administration (FDA) declined to approve MDMA-assisted therapy for the treatment of post-traumatic stress disorder (PTSD).1 The agency cited insufficient data and requested that Lykos Therapeutics, the company seeking approval, conduct an additional clinical trial to thoroughly assess the drug’s safety and efficacy.1 This requirement could add years to the approval process and cost millions of dollars. The setback has prompted the psychedelic research community to reassess the current methodologies for studying psychedelic therapies, including the reliance on controversial preclinical models.
![“The failure of MDMA therapy to gain regulatory approval for clinical use indicates that the path forward for [psychedelic assisted therapy] will be highly scrutinized. … For any of these to become a breakthrough therapy, the scientific and biomedical community needs to do a better job of building confidence about the data coming from these trials.” —S.B. Armstrong and A.K. Davis, doi/10.1126/science.adt1024](https://www.scienceadvancement.org/wp-content/uploads/2024/10/quote-box.jpg)
Psychedelic research is being explored as a treatment for various conditions, including major depression,2 anxiety,3 eating disorders,4 addiction,5 and PTSD. However, psychedelic drug development is at risk of falling into the same pitfalls common to psychiatric drug research: insufficient internal and external validity in preclinical research.
Ferreira et al. (2023) explored the high failure rate of drug development programs for psychiatric diseases in phase II and phase III trials, which they attribute to the poor translatability of preclinical psychiatric drug experiments to clinical studies.6 Many animal-based preclinical studies fail to report critical information, such as blinding, randomization, the animals’ sex and housing conditions, or route of administration.6 They propose that such poor reporting often leads to an overestimation of the predictive value of experiments on animals, allowing first-in-human trials to proceed based on incomplete or flawed preclinical data.6 In a 2023 perspective, Paul and Potter argue that the use of animal models for psychiatric disorders requires a “leap of faith” in which researchers assume that observed behaviors in animals can accurately reflect complex human psychiatric conditions.7 Paul and Potter contend that psychiatric drug development has lagged behind other areas of medicine, with most current treatments for mood and anxiety disorders being no more effective than those introduced over 50 years ago.7
The Wrong Models
A key reason for this stagnancy is the heavy reliance on experiments that use animals to attempt to mimic psychiatric disorders.7 One example of an animal model that fails to mimic a psychiatric disorder is the forced swim test for depression—which has been widely criticized for its cruelty and lack of effectiveness.8,9 This criticism has led international government funding agencies, such as the National Health and Medical Research Council in Australia, to no longer provide funding for studies using the forced swim test for many endpoints.10 It has also the led the Australian state of New South Wales to make it entirely illegal and the U.S. National Institute of Mental Health to discourage its use.10,11,12
In psychedelic drug research, the head twitch response (HTR) test is often used to measure rapid, rhythmic head movements in mice or rats after they have been given psychedelic drugs that target a specific brain receptor.13 Although some experimenters claim the test can predict psychedelic effects in humans, some drugs produce false positives.13
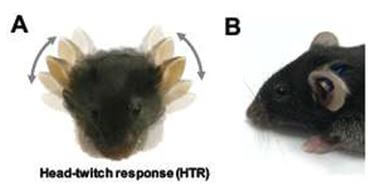
Adding injury to insult, the test involves invasive procedures: Experimenters cut into an animal’s scalp in order to place a magnet on their skull, securing it with dental cement, to automize data collection.14 Additionally, experimenters may restrain and immobilize a mouse’s head in order to pierce their ear with a magnet without anesthesia.15 Experimenters assert that the HTR test can help determine if a compound reaches the brain after systemic administration.13 However, the behavior being measured doesn’t correlate with any meaningful symptoms of human psychiatric disorders, as humans don’t exhibit head twitches when they’ve taken psychedelics.13

Human Problems Need Human Solutions
Ethical and effective human-relevant alternatives for determining drug delivery to the human brain already exist. For example, researchers are using in vitro techniques, ranging from traditional 2D cell cultures to 3D tissues. For example, researchers using organs-on-chips can replicate the physiological functions of the blood-brain barrier while also maintaining viability for weeks, allowing them to study long-term effects.16 This technology has been used to assess drug delivery to the brain for psychiatric and neurological conditions, such as Alzheimer’s disease.17 Continued investment in poor models diverts essential resources—funding, researchers, and time—from developing more effective psychedelic drug preclinical models and hinders medical progress and decision-making.
More funding and resources must be directed toward preclinical models that use human cells and data to evaluate the delivery of psychedelic drugs without invasive procedures on animals. Noninvasive, human biology–based models allow researchers to replicate studies more readily and to verify previous conclusions—a hallmark of good science that is often not possible with experiments on animals. Additionally, these reliable models serve as a consistent benchmark, enabling scientists to make more accurate comparisons of newly developed drugs and ensuring that future studies build on standardized, validated methods.
Challenging Assumptions
In clinical psychiatry, many medications are developed and prescribed without a comprehensive understanding of their mechanisms of action. Instead, physicians often rely on observed associations between the use of certain drugs and improved patient outcomes, operating under the assumption that offering a treatment, even if it’s not fully understood, is preferable to providing none. For example, the mechanisms of action for many antidepressants—among the most prescribed psychiatric medications—aren’t fully understood.
One of the most influential assumptions about the etiology for depression has been the serotonin hypothesis,18 which posits that depression is caused by lower levels of serotonin in the brain. This theory continues to be taught to doctors-in-training and explained to patients.18 Much of the foundation for the serotonin hypothesis is derived from experiments on animals in which researchers attempted to correlate animal behaviors with depressive symptoms in humans and then associate these behaviors with varying serotonin levels.19 However, a 2023 systematic review by Moncrieff et al. examining human-based studies of depression and serotonin levels concluded that there is no consistent evidence to support an association between the two.18 Given the significant investment of resources and patients’ reliance on researchers to use the best available tools to develop effective treatments, a critical reevaluation of current approaches to drug development for depression and other psychiatric conditions—including the use of psychedelics—is warranted.
Future researchers in neuroscience and psychiatric domains need to actively consider how the practice of extrapolating animal behaviors to human symptomatology has resulted in a distorted understanding of disease pathology. This approach leads to searches for biochemical markers that align with inaccurate models. Consequently, future researchers may continue using animal models for the sake of consistency with older data, perpetuating a cycle that further degrades understanding of the disease. This cycle significantly hampers the ability to translate findings into meaningful clinical research.
Revitalizing the Revolution
The psychedelic research community and its advocates must push for increased funding to implement and continue to develop non-animal models for studying psychedelic treatments. Evidence-based policies should redirect the field’s focus to human-based experimental models, such as computational modeling, advanced in vitro experiments (including those using organs-on-a-chip, human brain organoids, and patient-specific stem cells for personalized medicine), human neuroimaging, and human omics research. These new approaches will help pave the way for more reliable and ethical advancements in psychedelic drug development for psychiatric disorders.
If psychedelic research doesn’t evolve, it risks again being relegated to the fringes of scientific inquiry. As we push boundaries in understanding the human mind, being “shaken out of the ruts of ordinary perception,”20 we must prioritize human-based research over archaic and misleading animal models and ensure that the methods used for studying these powerful compounds are equally innovative and grounded in human experience. Championing human-relevant research in this field enhances the efficacy of the science and honors the ethical considerations that should guide it.
1Lykos Therapeutics. Lykos Therapeutics announces complete response letter for midomafetamine capsules for PTSD. Published August 9, 2024. Accessed August 19, 2024. https://www.prnewswire.com/news-releases/lykos-therapeutics-announces-complete-response-letter-for-midomafetamine-capsules-for-ptsd-302219182.html
2Ko K, Kopra EI, Cleare AJ, Rucker JJ. Psychedelic therapy for depressive symptoms: a systematic review and meta-analysis. J Affect Disord. 2023;322:194-204. doi:10.1016/j.jad.2022.09.168
3Muttoni S, Ardissino M, John C. Classical psychedelics for the treatment of depression and anxiety: a systematic review. J Affect Disord. 2019;258:11-24. doi:10.1016/j.jad.2019.07.076
4Calder A, Mock S, Friedli N, Pasi P, Hasler G. Psychedelics in the treatment of eating disorders: rationale and potential mechanisms. Eur Neuropsychopharmacol. 2023;75:1-14. doi.org/10.1016/j.euroneuro.2023.05.008
5Zafar R, Siegel M, Harding R, et al. Psychedelic therapy in the treatment of addiction: the past, present and future. Front Psychiatry. 2023;14:1183740. doi:10.3389/fpsyt.2023.1183740
6Ferreira GS, Dijkstra FM, Veening-Griffioen DH, et al. Translatability of preclinical to early clinical tolerable and pharmacologically active dose ranges for central nervous system active drugs. Transl Psychiatry. 2023;13(1):74. doi.org/10.1038/s41398-023-02353-1
7Paul SM, Potter WZ. Finding new and better treatments for psychiatric disorders. Neuropsychopharmacol. 2024;49:3-9. doi.org/10.1038/s41386-023-01690-5
8Trunnell ER, Carvalho C. The forced swim test has poor accuracy for identifying novel antidepressants. Drug Discov Today. 2021;26(12):2898-2904. doi.org/10.1016/j.drudis.2021.08.003
9Trunnell ER, Baines J, Farghali S, Jackson T, Jayne K, Smith R, Stibbe T. The need for guidance in antidepressant drug development: revisiting the role of the forced swim test and tail suspension test. Regul Toxicol Pharmacol. 2024;151:105666. doi.org/10.1016/j.yrtph.2024.105666
10Reardon S. Pressure grows to ditch controversial forced swim test in rodent studies of depression. Science. Published March 20, 2024. Accessed August 19, 2024. https://www.science.org/content/article/pressure-grows-to-ditch-controversial-rodent-test-in-depression-studies#:~:text=In%20December%202023%2C%20Australia’s%20National,FST%20entirely%20illegal%20in%20December.
11National Health and Medical Research Council. Statement on the forced swim test in rodent models. Published December 13, 2023. Updated January 24, 2024. Accessed August 20, 2024. https://www.nhmrc.gov.au/research-policy/ethics/statement-forced-swim-test-rodent-models.
12Australian Government. Animal Research Amendment (Prohibition of Forced Swim Tests and Forced Smoke Inhalation Experiments) Bill 2024. Parliament of New South Wales. Assented on March 25, 2024. Accessed August 20, 2024. https://www.parliament.nsw.gov.au/bills/Pages/bill-details.aspx?pk=18431.
13Odland AU, Kristensen JL, Andreasen JT. Animal behavior in psychedelic research. Pharmacol Rev. 2022;74(4):1176-1205. doi:10.1124/pharmrev.122.000590
14Halberstadt AL, Luethi D, Hoener MC, Trachsel D, Brandt SD, Liechti ME. Use of the head-twitch response to investigate the structure-activity relationships of 4-thio-substituted 2,5-dimethoxyphenylalkylamines. Psychopharmacology (Berl). 2023;240(1):115-126. doi:10.1007/s00213-022-06279-2
15de la Fuente Revenga M, Vohra HZ, González-Maeso J. Automated quantification of head-twitch response in mice via ear tag reporter coupled with biphasic detection. J Neurosci Methods. 2020;334:108595. doi.org/10.1016/j.jneumeth.2020.108595
16Humpel C. Organotypic brain slice cultures: a review. Neuroscience. 2015;305:86-98. doi:10.1016/j.neuroscience.2015.07.086
17Yoon JK, Kim J, Shah Z, Awasthi A, Mahajan A, Kim Y. Advanced human BBB-on-a-chip: a new platform for Alzheimer’s disease studies. Adv Healthc Mater. 2021;10(15):e2002285. doi:10.1002/adhm.202002285
18Moncrieff J, Cooper RE, Stockmann T, et al. The serotonin theory of depression: a systematic umbrella review of the evidence. Mol Psychiatry. 2023;28:3243–3256. doi.org/10.1038/s41380-022-01661-0
19Stanford SC. Confusing preclinical (predictive) drug screens with animal “models” of psychiatric disorders, or “disorder-like” behaviour, is undermining confidence in behavioural neuroscience. J Psychopharmacol. 2017;31(6):641-643. doi:10.1177/0269881116689260
20Huxley A. The Doors of Perception. Harper & Row; 1954.
